I wanted to share a note on the SMA isotherm with reference concentrations because I got a bit tripped up with this.
I have found using reference concentrations to be helpful because without them I have found some simulations tend to get stuck, and using the reference concentrations as recommended (c_ref = maximum inlet concentration of the mobile phase modifier and q_ref = ionic capacity) solves that problem.
The CADET documentation for reference concentrations found here does explain how parameter transformation works, but I initially incorrectly assumed that this was a transformation that occurs internally and that the ka and kd parameters entered by the user corresponded to the actual ka and kd parameters rather than the transformed ones. I think this is currently a bit unclear from the documentation.
I found this post from 2023 to be helpful in clarifying that the parameters entered by the user and fit during optimization correspond to the transformed parameters rather than the actual ones, and I agree with @j.schmoelder that this is not ideal.
Additionally, this note documents the introduction of the option to use untransformed rate constants in CADET-Process, which is helpful if you know it exists and when to use it. However, I found that you have to be careful about using the untransformed parameters because the reference concentrations must be specified before the parameters are set.
This works:
binding_model.reference_liquid_phase_conc = c_ref
binding_model.reference_solid_phase_conc = q_ref
binding_model.adsorption_rate_untransformed = [0, ka]
binding_model.desorption_rate_untransformed = [0, kd]
While this does not:
binding_model.adsorption_rate_untransformed = [0, ka]
binding_model.desorption_rate_untransformed = [0, kd]
binding_model.reference_liquid_phase_conc = c_ref
binding_model.reference_solid_phase_conc = q_ref
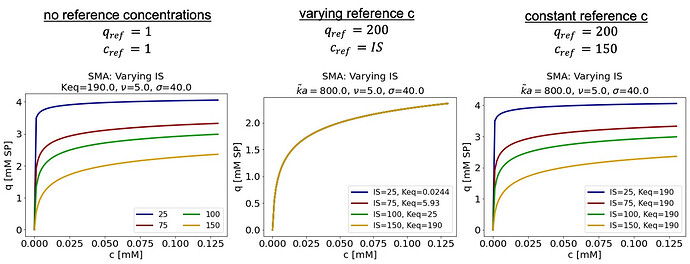
Lastly, when fitting ka transformed to data from multiple experiments at different ionic strengths, it does not work to use the ionic strength for each given experiment as the reference concentration. The reference concentration should be constant across all experiments. If the reference concentration isn’t constant, even though the transformed ka and kd are constant, the actual kd is not constant, so the actual Keq is not constant.
| c_ref | q_ref | 𝜈 | ka (trans) | kd (trans) | ka (actual) | kd (actual) | Keq |
|---|---|---|---|---|---|---|---|
| 25 | 200 | 5 | 800 | 1 | 2.5E-09 | 1.02E-07 | 0.0244 |
| 75 | 200 | 5 | 800 | 1 | 2.5E-09 | 4.21E-10 | 5.93 |
| 100 | 200 | 5 | 800 | 1 | 2.5E-09 | 1.00E-10 | 25.0 |
| 150 | 200 | 5 | 800 | 1 | 2.5E-09 | 1.32E-11 | 190 |
This leads to the isotherm shown in the middle rather than an isotherm that varies with ionic strength as expected.
This makes sense knowing that the parameter transformation is not internal and that the parameters being fit are the transformed ka and kd rather than the actual ka and kd. But, if like me you didn’t initially realize how reference concentrations work, this could be confusing, which is why I wanted to write this post. ![]()